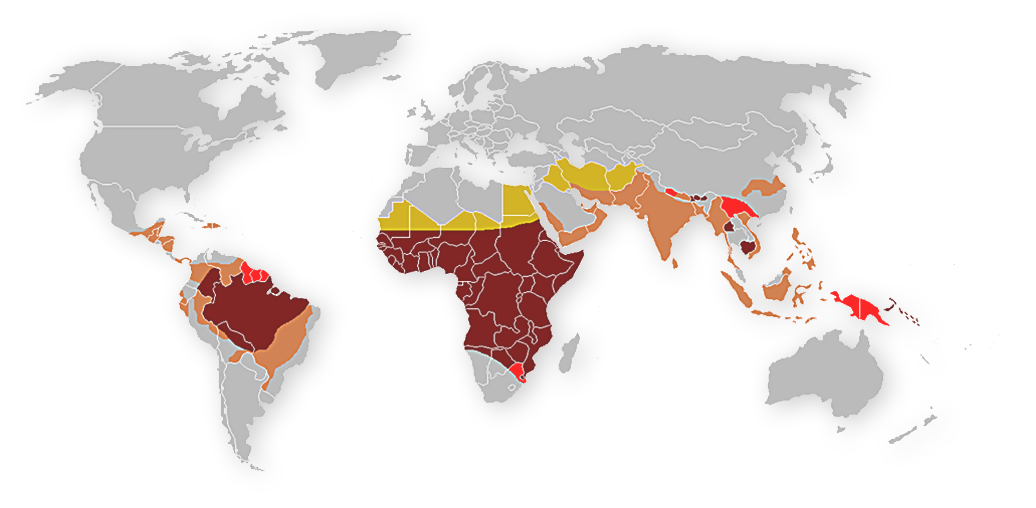
What MALARIA means
Data from: World Malaria Report 2020
50%
of the world population at risk of infection
82%
of cases was in Africa Region
94%
of deaths was in Africa
229 M
cases in 2019
409.000
deaths
67%
of deaths was children under 5 years
WHO recommends prompt parasite-based diagnosis in all patients suspected of malaria before treatment is administered. Malaria rapid diagnostic tests have the potential to greatly improve the quality of management of malaria infections, especially in remote areas with limited access to good quality microscopy services.

Rapid Diagnostic Test market size
Data from: World Malaria Report 2020
|
|
global market346 M RDT sold in 2019 |

concept
Malaria disease is caused by the Plasmodium parasite, which attacks red blood cells. During the intra-erythrocytic development Plasmodium feeds on hemoglobin, degrading it into hemozoin nanocrystals. Being paramagnetic, these crystals allow for the magnetic separation of infected red blood cells from healthy ones.
The diagnostic test TMek exploits this property to quantify infected red blood cells:
1. A small drop of blood is placed in a small chamber.
2. A microchip with micron-sized magnetic concentrators and sending electrodes, is used to seal the chamber and placed in vertical position, in the magnetic field produced by an external magnet.
3. Healthy RBC and other corpuscles sediment while i-RBC are captured on the surface of concentrators, where the sensing electrodes allow for their quantification.
4. The signal is displayed on a notebook or smartphone connected to the diagnostic apparatus.
TIME-SAVING
QUANTITATIVE
METHOD
PANPLASMODIC
SENSITIVITY
SINGLE DROPLET
ON-CHIP
NO QUALIFIDED
PERSONNEL
Competiton
Need of a compact, low-cost and easy to use diagnostic system, which allows a rapid detection, with the same sensitivity of the Gold Standard
World Health Organisation


State of the art of diagnostic tests
GS: It consists of the direct microscopic parasite investigation performed both on a thick and on a thin blood film stained on a glass slide. The evaluation is performed through the count of the number of infected RBCs over different fields of view and the result is expressed with respect to the total number of RBCs evaluated (parasitaemia%). The limit of detection (LoD) ranges from 4 to 20 parasites/μL, but the operational time required for the analysis is around 30 to 60 minutes. Moreover the accuracy is user dependent.
RDT: Antigen-detecting RDT technology it is considered to be a significative alternative to the gold standard. The solution proposed by the RDT technology is cheap and easy to use. The test is fast compared to the gold standard, requiring only 20 minutes, and it can reach a LoD that is around 200 parasites/μL. Critical aspects of RDTs are the presence of a sizable number of false positive and negative results, as well as the inability to quantify the parasitaemia, leading to a non quantitative diagnostic test.
PCR: is based on the detection of specific nucleic acid sequences. It is an enzymatic assay which amplifies a specific segment of a DNA template by creating thousands of copies of that particular sequence. It presents the highest sensitivity and specificity that both reaches 100% for parasitaemia levels of few parasites/μL (0,0001%) and, in addition, it is able to detect all kind of plasmodium species. High costs, long operation time, and the fact of being a qualitative test in the version suitable for on field application, are the critical aspects of this technology.
Among other advantages, TMek is faster than any diagnostic test currently on the market (it takes just 7 minutes) and offers the unique feature of allowing for the automatic quantification of the parasitaemia.
MISSION
Our mission is developing diagnostic technologies suitable for on-site use in endemic zones, like the rapid diagnostic test TMek for malaria. The initial project “Tid Mekii” (from the name of malaria in Camerun), originates from the social program “Polisocial awards” of Politecnico di Milano. In this spirit, we invented the concept of “social patent”, i.e. a form of protection of the intellectual property against a pure economic deployment.
Our business model is based on the principles of corporate ethics, with the aim of conjugating high technology, sustainable economy and social needs.
SOCIAL PATENT
The nature of the Polisocial Awards grant which made possible the research on TMek, forced us to think about an unusual kind of protection of the intellectual property: the “social patent”. The scope was not protecting our intellectual properties to beneficiate from possible revenues or other economic initiatives. The idea was to protect the invention against a pure economic deployment. Malaria is a serious medical and social problem; our invention couldn’t become just a way of transforming it in a business.
For this reason, in agreement with the TTO of Politecnico di Milano, we signed an internal document preliminary to the patent application where the inventors:
1. Give up all possible revenues from the exploitation of the IP, while declaring their interest in continuing the development of the diagnostic test.
2. Requests Politecnico di Milano adopting a no-profit exploitation strategy, compatible with the principles of responsible, sustainable and fair development, as well as attentive to local and global social needs. This was the ideation of the “Social Patent”.
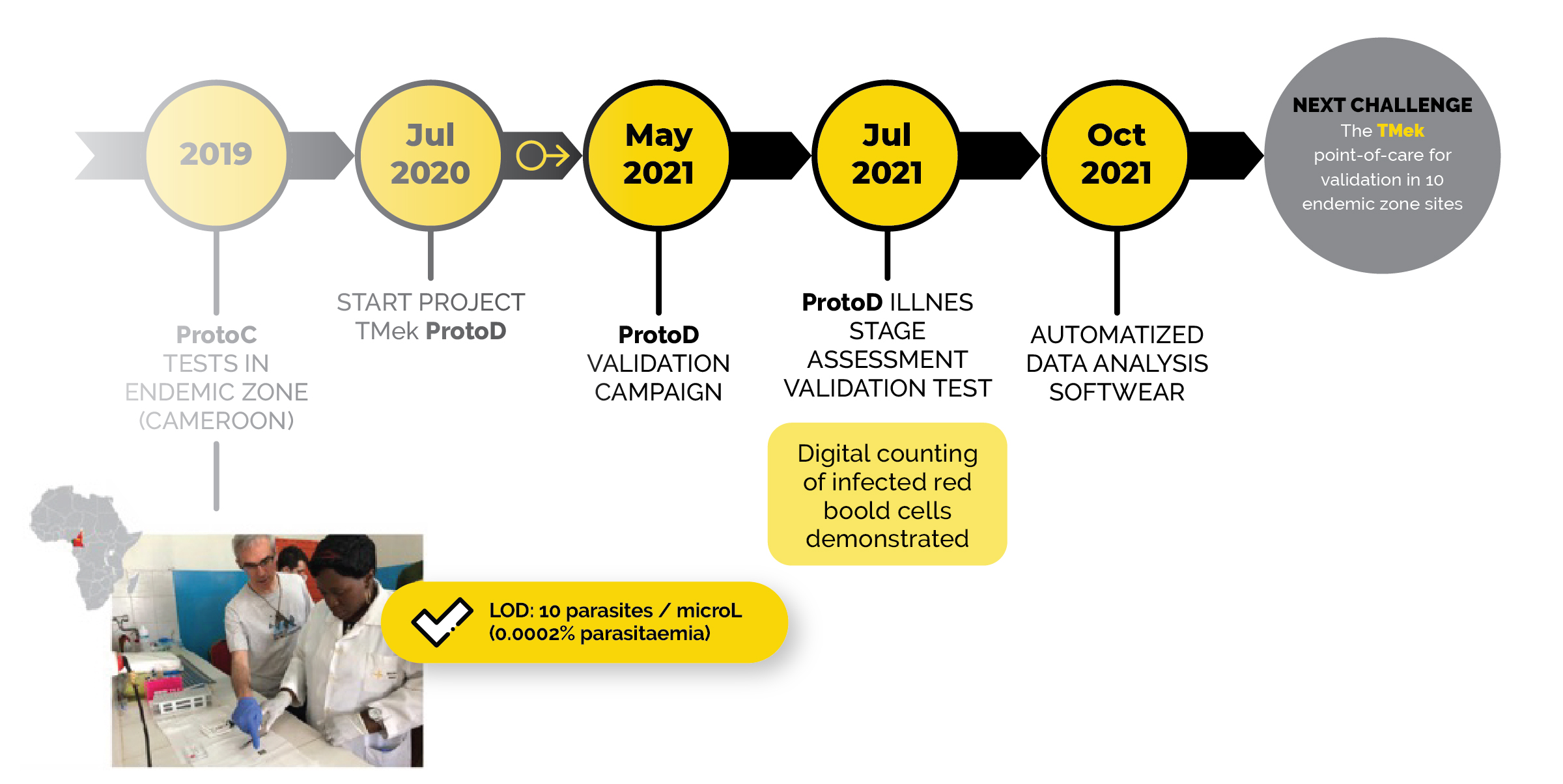
Where we are

S2P competition 2018
WINNERS
Disruptive Innovation Award
features after the first validation
-
Direct and automatic quantification of the parasitaemia
-
Quantification of circulating malaria pigment
-
Panplasmodic test
-
Suitable for post treatment monitoring of illness evolution
-
Specimen: 7 μl of whole blood from a prick test
-
Test operating time: 7 minutes
-
Storage conditions: -20°C – 50°C (to be refined)
-
Sensitivity: 100% (93.3-100) (venous sampling) – 100% (63.0-100) (capillary sampling)
-
Specificity: 69% (49.2-84.7) (venous sampling) – 100% (capillary sampling)
-
LOD: 10 parasites / μl (0.0002% parasitaemia)
Development plan/1
Who we are
Academic staff

Riccardo Bertacco
Coordinator
Full Professor
Dept. of Physics – Polifab

Giorgio Ferrari
Development of the integrated electronics
Researcher– Electronic Eng.

Gianfranco Beniamino Fiore
Hematic treatments
Associate Professor
Biomedical Eng.
…past members

Jonathan Barsotti
Electrical measurements
Data Analysis
Test Optimization
Physicist
PhD on Organic Electronics

Francesca Milesi
Chip production
Test optimization
PhD Candidate in Physics Engineering

Lorenzo Coppadoro
Hematic treatments
Mechanical setup
PhD Candidate in Biomedical Eng.

Tommaso Pravettoni
Electrical measurements
Chip Production
Physics Engineering Master Student

Cainã de Oliveira Figares
Integrated Electronics
Firmware
Electronic Engineering Master Student
Our partners

Divisione IIIª di Malattie Infettive dell'Università di Milano.
Doctors and researchers of Divisione IIIª of the Infective Diseases section of the University of Milano (Ospedale Sacco)
Prof. Spinello Antinori
Dott. Romualdo Grande

Hopital Saint Luc de Mbalmayo
Dott. Paul Tina (Director)
Joel Bombe (Diagnostic Lab. Manager)
ONG COE
Rosa Scandella
(Outgoing President)
Publications
Giacometti M., Rinaldi C., Monticelli M., Callegari L., Collovini A., Petti D., Ferrari G., Bertacco R.,
“Electrical and magnetic properties of hemozoin nanocrystals”.
Applied Physics Letters, 113(20), 203703 (2018).
https://doi.org/10.1063/1.5050062
“On-Chip Selective Capture and Detection of Magnetic Fingerprints of Malaria”.
Sensors 2020, 20, 4972.
https://doi.org/10.3390/s20174972
Giacometti M., Milesi F., Coppadoro P.L., Antinori S., Bertacco R.,
“A Lab-On-chip Tool for Rapid, Quantitative, and Stage-selective Diagnosis of Malaria”.
Advanced Science, 2021, 8(14), 2004101.
https://doi.org/10.1002/advs.202004101
Giacometti M., Monticelli M., Piola M., Fiore G.B., Bertacco R.
“On-chip magnetophoretic capture in a model of malaria-infected red blood cells”.
Biotechnology and Bioengineering, 2022, 119(4), pp. 1129–1141
https://doi.org/10.1002/bit.28030
Contact
Prof. Riccardo Bertacco
Department of Physics – Politecnico di Milano
Via Giuseppe Colombo 81, 20133 Milano (Italy)
tel. +39.02.2399.9663
info@tmekdiagnostics.com
Prof. Giorgio Ferrari
DEIB – Politecnico di Milano
Via Giuseppe Colombo 81, 20133 Milano (Italy)
info@tmekdiagnostics.com




